UBC(不列颠哥伦比亚大学)医学院的研究人员对Omicron变种刺突蛋白进行了世界上第一次分子水平的结构分析。研究结果发表在今天的《科学》杂志上。Cryo-EM用低温电子显微镜以接近原子分辨率完成——揭示了严重突变的Omicron变种是如何附着并感染人类细胞的。
Omicron变种刺突蛋白的原子结构(图片)

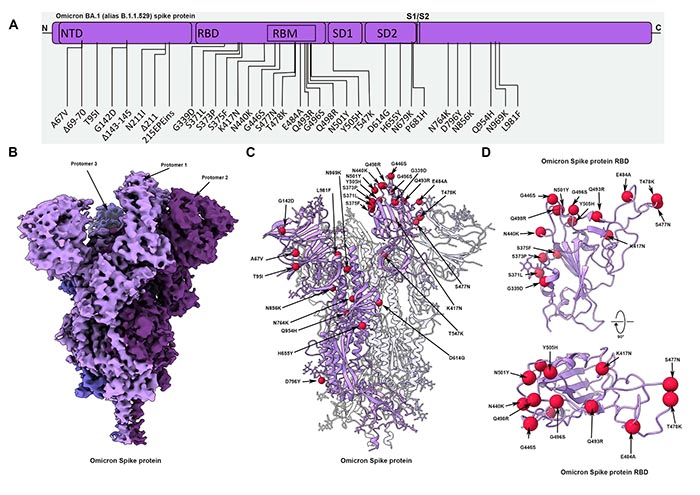
位于冠状病毒外部的刺突蛋白使SARS-CoV-2能够进入人类细胞。Omicron变种在其刺突蛋白上有前所未有的37个突变,比以前的变种多出3到5倍。
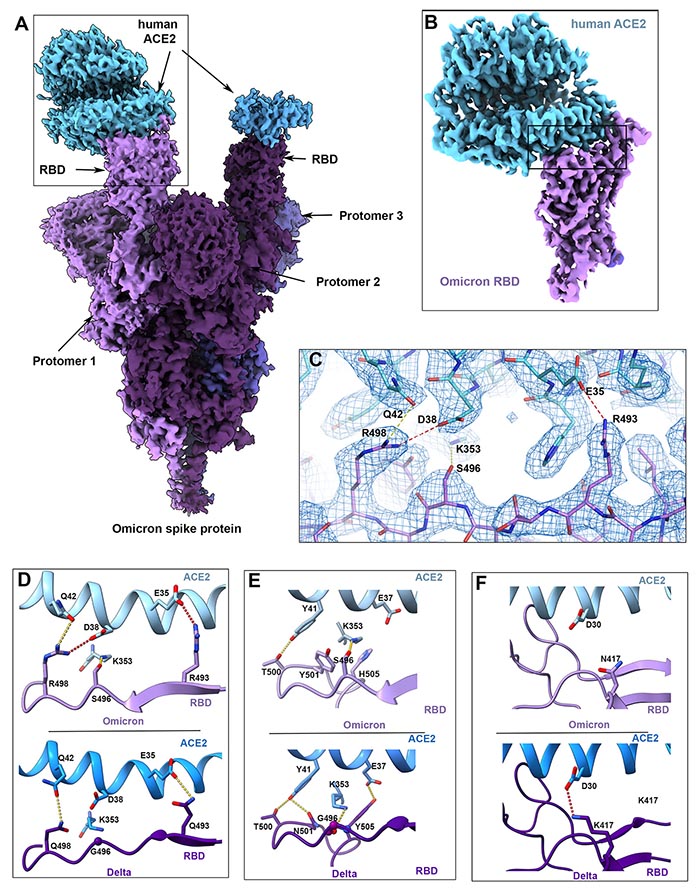
结构分析显示,一些突变(R493, S496和R498)在刺突蛋白和人类细胞受体ACE2之间建立了新的盐桥和氢键。研究人员得出结论,这些新的结合似乎增加了结合的亲和力,即病毒与人类细胞的结合强度,而其他的突变(K417N)则降低了这种结合的强度。
Cryo-EM structural analysis of the Omicron spike protein ectodomain shows that the overall organization of the trimer is similar to that observed for the ancestral strain (5–7) and all earlier variants (8–10) (Fig. 1B and table S1). The RBD in one of the protomers (protomer 1) is well-resolved and is in the “down” position, while the other two RBDs are less well-resolved because they are flexible relative to the rest of the spike protein polypeptide. Similarly, the amino terminal domain (NTD) is poorly resolved, reflecting the dynamic and flexible nature of this domain. The mutations in the Omicron variant spike protein are distributed both on the surface and the interior of the spike protein (Fig. 1C), including NTD and RBD regions. The mutations in the RBD are predominantly distributed on one face of the domain (Fig. 1D), which spans regions that bind ACE2 as well as those that form epitopes for numerous neutralizing antibodies (11). The Omicron variant shares RBD mutations in common with previous variants of concern (K417N, T478K, and N501Y). The N501Y and K417N mutations impart increased and decreased ACE2 binding affinities respectively (8, 12–16). These mutational effects preserve the same general impact on ACE2 affinity when present in isolation or in combination with other RBD mutations (12). However, the Omicron RBD contains additional mutations, the majority of which have been shown to decrease receptor binding in a high-throughput assay (table S2) (17), with the exception of G339D, N440K, S447N, and Q498R (17, 18). To measure the impact of Omicron spike protein mutations on human ACE2 binding affinity, we performed surface plasmon resonance studies and compared the resulting apparent binding affinities (KD,app) to wild-type and Delta spikes (Fig. 2). Wild-type (WT) is used in this work to refer to the ancestral Wuhan-Hu-1 strain with the addition of the D614G mutation. While the Omicron spike protein exhibits a measurable increase in apparent affinity for ACE2 relative to the wild-type spike [in agreement with a recent preprint (19)], the apparent ACE2 affinity is similar for Delta and Omicron variants (Fig. 2D). Despite harboring several RBD mutations which decrease ACE2 binding (fig. S2) (12, 16, 17), the preservation of overall ACE2 binding affinity for the Omicron spike protein suggests there are compensatory mutations that restore higher affinity for ACE2. Such mutational effects should be possible to visualize in a high-resolution structure of the spike protein-ACE2 complex.
Subramaniam博士说:“总的来说,研究结果表明,Omicron比原始病毒具有更大的结合亲和力,其水平与我们所看到的Delta病毒变种更相似。”“值得注意的是,尽管有如此广泛的突变,Omicron变种进化后仍然保持了与人类细胞结合的能力。”
研究人员进行了进一步的实验,表明Omicron刺突蛋白表现出增加的抗体逃避。与之前的变种相比,Omicron在所有六种单克隆抗体测试中显示出可测量的逃避,其中五种单克隆抗体完全逃避。该变异还显示了从接种疫苗的个人和未接种的COVID-19患者收集的抗体的逃避增加。
值得注意的是,与未接种疫苗的患者自然感染产生的免疫力相比,Omicron对疫苗产生的免疫力的规避更少。这表明接种疫苗仍然是我们最好的防御手段。”Subramaniam博士说。
根据观察到的结合亲和力和抗体规避的增加,研究人员说刺突蛋白突变可能是增加Omicron变异的传播能力的因素。
Subramaniam博士说,下一步,他的研究团队将利用这些知识来支持更有效治疗方法的开发。
“我们团队的一个重要重点是更好地了解中和抗体的结合,以及在整个变异范围内有效的治疗方法,以及如何利用这些方法来开发抗变异治疗。”
https://www.science.org/doi/10.1126/science.abn7760













